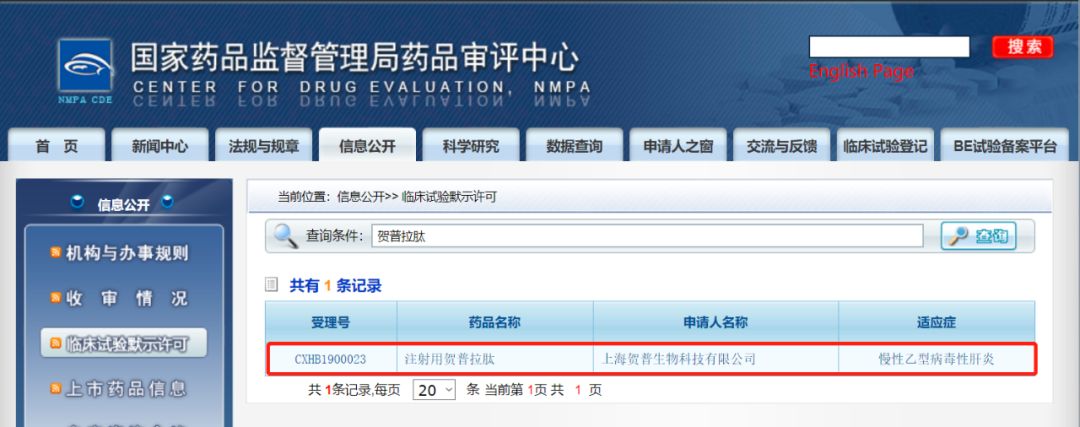
Hepatite, a new hepatitis B drug entry inhibitor, is approved for Phase II / III clinical trials
According to the news from the website of the Drug Evaluation Center of the State Drug Administration, the first-in-class hepatitis B new drug Hepalatide developed by Shanghai Hepu Pharmaceutical Co., Ltd. has obtained the default license of NMPA clinical trials and will Phase II / III clinical development stage.
"None of the more than 5,000 existing pharmaceutical companies in the country have yet entered the world's top 50 pharmaceutical companies. Even from the perspective of generic drugs, Chinese pharmaceutical companies are quite far from generic drug giants such as Israel's Teva and Germany's Sandoz." In a recent corporate interview, Dr. Hongli Liu, General Manager of Shanghai Hepu Biotechnology Co., Ltd., was worried about this. 90% of the drugs currently approved in China are dosage form modification and generic drugs. The main reason is that the investment in innovative drug research and development is long and the research and development cycle is long. The majority of pharmaceutical companies may be discouraged or unwilling to take risks. Among the only 10% innovative drug research and development companies, some companies did not proceed from market demand, and the variety development process did not form a capitalized business development model, resulting in the failure of most innovative varieties to effectively industrialize and eventually die.
Hepalatide is an entry inhibitor that blocks HBV infection by binding to the HBV hepatocyte infection receptor NTCP, interrupting the long cycle of virus clearance → reinfection in existing hepatitis B treatments. Healthy liver cells will gradually complete the replacement of HBV infected liver cells, thereby improving the treatment efficiency of chronic hepatitis B, and may even make chronic hepatitis B completely cured.
Hepalatide has obtained the approval for the clinical trial of Class 1.1 drugs in November 2014 and has completed Phase I clinical research in Beijing 302 Hospital.
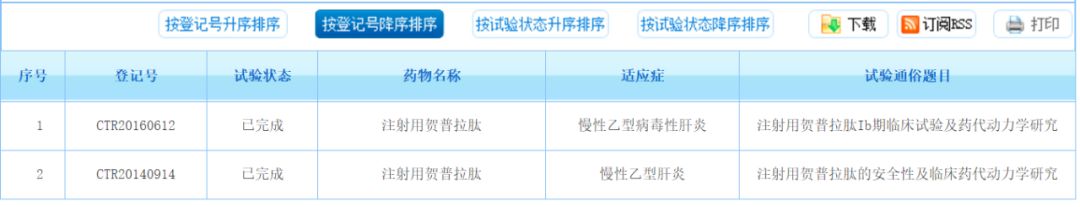
At the previous second China Pharmaceutical Innovation Investment Conference held in Suzhou, Liu Heli, CEO of Shanghai Hepu Pharmaceutical Co., Ltd., made the first announcement of the clinical research data of heparatide.
The evaluation of Hepalatide to block HBV infection in vivo showed that the HBV DNA (copy / ml) level fell below the detection limit after 9 days of treatment in the high and medium dose groups, and the serum ALT (IU / ml) The level drops below the detection limit. Pharmacokinetic studies have shown that hepalatide has significant liver targeting.
Randomized, double-blind, blank-controlled dose-increasing phase I clinical trials of hepalatide for injection, for patients with positive anti-HBV Pre-S1 antibody positive, 4: 1 double-blind, random access to the trial and control group. There were 45 patients enrolled in Phase Ia and 35 patients in Phase Ib. The clinical trial results showed that the drug was excellent in safety, obtained the clinically recommended dose and verified that the bile acid elevation (TBA) was dose-dependent.
The Phase II / III clinical study to be launched will take the sustained virological response after drug withdrawal as the main end point. Academician Wang Fusheng of the Fifth Medical Center of the PLA General Hospital served as the principal investigator of the clinical trial. The company expects to apply for the listing of new drugs after completing Phase III clinical research in 2022.
The basic structure of Heparatide was designed by Hepu Pharmaceutical R & D team based on the popular C genotype HBV Pre-S1 in China. Its patent application was earlier than the international MyrcludexB which also originated from the amino acid sequence of C genotype HBV Pre-S1 It belongs to the first-in-class HBV virus entry inhibitor new drug.
In 2018, the National Drug Administration (NMPA) was the first in the world to publish the "Technical Guidelines for Clinical Trials of Chronic Hepatitis B Antiviral Therapeutics", which determined the end point of clinical research with a sustained virological response and surface antigen negative conversion Provide timely standardization and guidance for the clinical development of innovative drugs for hepatitis B.
In November 2012, the sodium-cholic acid transporter NTCP was identified as a HBV infection receptor by the Chinese scientist Dr. Li Wenhui. This achievement is a key milestone in the field of hepatitis B research. The determination of the hepatitis B infection receptor NTCP has fundamentally promoted a profound understanding of the mechanism of hepatitis B infection and will make a tremendous contribution to the prevention and treatment of hepatitis B.
The Phase II / III clinical study of Hepalatide has been supported by the national “Major New Drug Creation” major scientific and technological project. (For more liver disease new drug research information, please pay attention to the "Liver Time" WeChat public account)!
 Add:Room 601, Building 1, Lane 720 Cailun Road, Pudong New District, Shanghai
Add:Room 601, Building 1, Lane 720 Cailun Road, Pudong New District, Shanghai Tel:021-68412368
Tel:021-68412368 E-mail:xiongchunfang_hep@163.com
E-mail:xiongchunfang_hep@163.com